Introduction
Advances in multiple myeloma (MM) treatment improve survival but expose patients (pts) to significant financial toxicity (FT) due to lifelong administration of costly therapies. Financial support services (FSS) like nurse navigators (NN), financial advocates (FA), and social workers (SW) can offset healthcare costs by accessing patient assistance programs. However, these services are underused due to ad hoc referrals and reluctance to discuss financial issues. We developed a financial navigation program (FNP) that proactively identifies pts at risk of FT, comprehensively assesses their needs, systematically connects pts to resources, and follows-up to ensure needs are met. In a randomized controlled trial (RCT), we assessed whether this FNP reduces FT compared to usual care.
Methods
We recruited adult pts with MM receiving systemic treatment at the University of Pennsylvania at their follow-up appointments. Consenting pts completed the COmprehensive Score for financial Toxicity (COST) questionnaire; those at risk of FT (score<26) were randomized 1:1 to the intervention (FNP) or usual care. We aimed to randomize 82 participants to achieve 80% power based on prior studies (expected improvement in COST by 5 in intervention, 2 in usual care). Pts completed a baseline survey to assess socioeconomic and demographic factors, cost-coping behaviors, quality of life (QoL), and satisfaction. After 4 months, pts completed a similar, final survey. In the FNP, the NN assessed financial and transportation barriers to care by telephone, performed proactive outreach to FA and SW, and coordinated resources, followed by monthly follow-up calls. We measured patient-reported outcomes as change from baseline to study completion; the primary outcome was change in COST score. Secondary outcomes were changes in cost-coping behaviors, QoL, and satisfaction. We performed an intention-to-treat, bivariate analysis (t-test or χ 2 test) of each outcome and conducted rapid thematic analysis of free-text responses. We also observed consenting pts whose baseline COST≥26, and - in post-hoc analysis - assessed for differential change in COST score at follow-up among this cohort vs the randomized/at-risk population.
Results
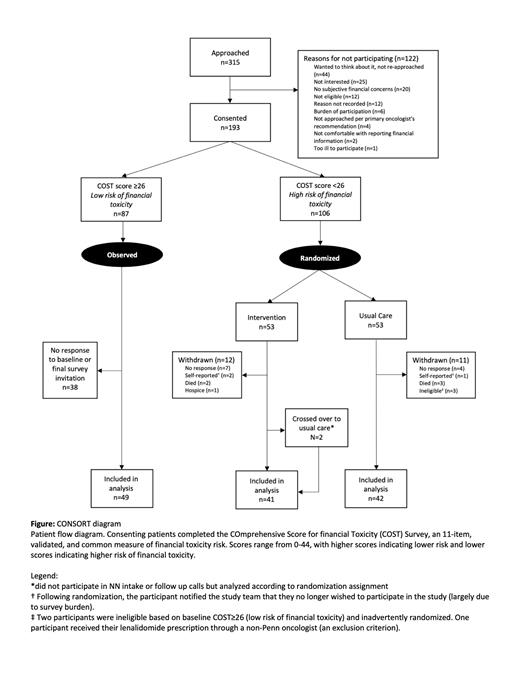
We assessed 315 pts from Feb-Dec 2022 ( Figure): 193 consented, 106 randomized, and 83 were included in analysis (41 in intervention, 42 in usual care). Randomized pts were an average of 65 years, 53% female, 39% nonwhite, 78% college-educated, and 51% earned ≤$60,000/year. This contrasted with 8% non-white pts among the non-randomized (high baseline COST) group (p<0.001). Despite all randomized pts being insured, 55% reported that MM treatment costs posed a significant financial burden. Many (46%) were on lenalidomide containing regimens. The mean, baseline COST score was 14.4 (SD 7.5) in the intervention versus 16.0 (SD 6.5) in usual care (p=0.3). Most pts had baseline FSS use, with a higher proportion in usual care (76%) than intervention (51%), p=0.03. During a median follow-up of 4.9 months, 17% in usual care received FSS. In the intervention arm, 95% participated in the FNP, with 68% completing ≥3 follow-up calls within 4 months. Randomized (at risk) pts showed an average improvement in COST of 4.6 (SD 8.9), significantly different from the non-randomized (low risk) group's mean worsening of -15.8 (SD 7.2) at follow-up; p<0.001. The intervention arm had a greater but non-significant improvement in COST score (mean 5.7 [SD 8.8]) compared to usual care (mean 3.5 [SD 8.9]); p=0.3. There were no significant changes in cost coping behaviors, QoL, or satisfaction between the two randomized arms. There was a significantly higher number of financial assistance applications submitted among pts randomized to the FNP (34%) vs usual care (12%); p=0.02. Patient feedback indicated the FNP improved understanding of FSS and reduced stress.
Conclusions
In this study, we confirmed that most pts with MM experience financial hardship and are at-risk of FT, despite being insured. We observed significant racial disparities in this risk. Our RCT showed that by screening pts with the COST tool and proactively coordinating FSS for at-risk pts, we can potentially reduce financial hardship. We attribute the non-significant improvement in FT to an under-powered analysis from higher-than-expected improvement in COST among usual care pts. Our program is efficient and scalable and demonstrated high retention.
Disclosures
Vogl:Karyopharm: Consultancy; Sanofi: Consultancy; GSK: Consultancy; Genentech: Consultancy; Active Biotech: Research Funding; Takeda: Consultancy, Research Funding. Cohen:Abbvie: Consultancy; Novartis: Patents & Royalties, Research Funding; Arcellx: Consultancy; Ichnos: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy; BMS/Celgene: Consultancy; Janssen: Consultancy, Research Funding; GSK: Consultancy, Research Funding; Genentech/Roche: Consultancy, Research Funding. Garfall:BMS: Consultancy, Honoraria; GSK: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Patents & Royalties, Research Funding; Legend: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Data Safety and Monitoring Board. Frosch:Seagen: Membership on an entity's Board of Directors or advisory committees; Roche: Research Funding; AstraZenica: Research Funding; Abbvie: Research Funding. Stadtmauer:Janssen: Consultancy; BMS: Consultancy; Abbvie: Consultancy, Research Funding; Amgen: Consultancy; genmab: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal